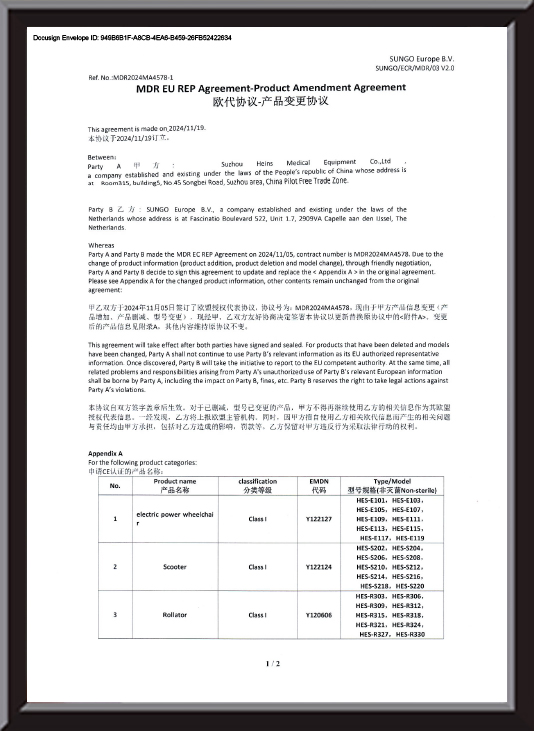
Custom Durable Electric Mobility Scooter Manufacturers
Mobility Scooter are a major technological innovation in modern transportation, offering a safe, comfortable, and efficient way to get around for those with limited mobility or anyone seeking convenience.
Built with advanced manufacturing processes and reliable electric drive systems, Mobility Scooter have become a familiar sight in daily life.
Structurally, an electric scooter typically consists of a durable frame, seat, control handle, tires, and core power and battery systems. The frame is crafted from high-strength, lightweight materials to ensure both stability and portability. The seat follows an ergonomic design, providing a comfortable experience even on longer rides.
These scooters offer a wealth of features beyond basic movement. Most of our models are equipped with lighting systems to ensure safety when traveling at night or in low-visibility conditions. Some high-end models also include adjustable seat heights and backrest angles to cater to the personalized needs of different users.
When it comes to target users, Mobility Scooter are especially loved by seniors, helping them easily handle daily errands, leisurely outings, and more. They also provide new independence for people with disabilities or those in rehabilitation, greatly improving quality of life and making travel simpler and more enjoyable.
-
Executive Summary In the domain of patient handling and mobility support, material selection is a central engineering decision impacting performance, ...
READ MORE -
Industry Background and Application Importance Global Mobility Needs and Travel Scenarios Mobility solutions play an essential role in enhancing the q...
READ MORE -
Industry Background and Importance of Application The aging global population and increasing demand for accessible healthcare mobility solutions have ...
READ MORE -
Industry Background and Application Importance The foldable electric wheelchair has become a critical mobility platform in healthcare, institutional, ...
READ MORE -
The aluminum alloy patient lifter is an essential device in modern healthcare settings, designed to assist in the safe transfer of patients with limit...
READ MORE
How to ensure that the power drive system of an electric scooter meets the durability standards of FDA and CE certification?
Core requirements of FDA and CE certification for the durability of the power drive system
FDA (U.S. Food and Drug Administration) classifies durable electric scooters as medical assistive devices. Its durability standards focus on safety, reliability and patient use scenario adaptability. It requires that the power system has no functional failures within the expected service life (usually 5-10 years), with particular attention to motor overheating protection, battery leakage prevention design and control system stability. CE certification (EU Conformity Certification) is based on the Machinery Directive (2006/42/EC) and the Low Voltage Directive (2014/35/EU), emphasizing mechanical strength, electrical safety and environmental adaptability. For example, the power system must pass the IP54 waterproof and dustproof test, the -20℃ to 50℃ temperature cycle test, and the motor's continuous operating life under rated load must be ≥10,000 hours.
The common requirements of the two include:
Material durability: key components must resist aging and corrosion and comply with the RoHS environmental protection directive;
Cycle life: the capacity retention rate of the battery pack after ≥1000 charge and discharge cycles is ≥80%;
Load stability: under the rated load (such as 150kg), the power output fluctuation range is ≤±5%;
Fault tolerance: the system must have overload protection, short circuit cutoff and fault warning functions.
Technical path and implementation strategy for durability assurance of power drive system
(I) Material and design optimization of core components
Durability construction of motor system
As the power core, the durability of the motor depends on the winding material, heat dissipation design and bearing life. For example, the use of NdFeB permanent magnets to improve the demagnetization resistance of magnetic steel, combined with a fully enclosed water-cooled heat dissipation structure, can control the motor operating temperature below 80°C (FDA requires the motor housing temperature to be ≤95°C). Suzhou Heins Medical Equipment Co., Ltd. incorporates patented technology into motor design. Its motor control system optimizes torque output through FOC vector control algorithm and reduces current impact during starting/braking. After testing, it can extend the life of motor bearings to more than 20,000 hours, meeting the long-term operation requirements of CE certification.
Battery system life management
The battery pack needs to be protected by both the thermal management system and the BMS battery management system. The battery module is wrapped with aviation-grade aluminum shell and carbon fiber composite material, which can not only improve the structural strength (resistance to 1000N extrusion without deformation), but also achieve ±2℃ temperature difference control through built-in thermal conductive silicone (FDA requires the battery operating temperature range of -10℃~45℃). BMS needs to monitor the single cell voltage, temperature and charge and discharge rate in real time. When the voltage of a battery cell deviates from the average value by ≥5%, the system automatically starts the balancing protection to avoid life attenuation caused by overcharging and over-discharging. The battery solution has been tested by a third party, and the capacity retention rate reaches 85% after 1C charge and discharge cycles for 1,000 times, which exceeds the basic requirements of CE certification.
Reliability design of controller and transmission system
The controller needs to use a three-proof paint coating (moisture-proof, dust-proof, and salt spray-proof) to meet the IP65 protection level to cope with outdoor humid environments. The transmission gearbox needs to pass the abrasive wear test. For example, the 20CrMnTi carburized and quenched gears are used, and the tooth surface hardness reaches HRC58-62. After 5000 hours of load operation, the tooth surface wear is ≤0.05mm. The transmission link design is optimized in the folding structure patent. By reducing the gear meshing clearance (≤0.02mm), the operating noise is reduced and the durability of the transmission system is improved.
(II) Quality control of production process and supply chain
Precision assurance of high-end manufacturing equipment
The processing accuracy of the core components of the power system directly affects the durability. For example, the German TRUMPF laser cutting machine used by Suzhou Heins Medical Equipment Co., Ltd. can achieve a cutting accuracy of 0.01mm for motor silicon steel sheets and reduce core loss; the Japanese Yaskawa robot welding station uses arc tracking technology to make the battery ear welding strength ≥50N, avoiding the increase of contact resistance caused by false welding (CE requires the temperature rise of the welding point ≤30K). The dust-free workshop (ISO8 level) of its 20,000 square meters modern production base can ensure that the impurity particles during the welding of the controller circuit board are ≤0.5μm, reducing the risk of short circuit.
Full-process traceability management of the supply chain
Key components (such as motor magnets and battery cells) must come from suppliers that have passed the IATF16949 certification, and each batch of materials must provide material certification and reliability test reports. For example, the battery cell must pass the UL1642 needle puncture test (no fire and explosion), and the motor bearing must provide a life certification of brands such as SKF or FAG (L10 life ≥50,000 hours). An efficient supply chain management system can achieve full-process traceability from raw material storage to finished product delivery, ensuring that each component of the power drive system meets the material compliance requirements of FDA and CE.
(III) Multi-dimensional testing system and certification compliance verification
Durability test simulating usage scenarios
Mechanical load test: Fix the robust mobility scooter on a vibration table, simulate rough road conditions with a frequency of 3Hz and an amplitude of ±50mm, and run continuously for 500 hours to test the fatigue strength of the motor bracket and battery bracket (FDA requires that the structural parts have no cracks and the bolts have no looseness).
Environmental cycle test: In a high and low temperature and humidity chamber, cycle at -20℃~50℃ (each temperature point is maintained for 8 hours), and apply 95% humidity for 100 cycles to verify the weather resistance of the controller's electronic components (CE requires that the insulation resistance after testing is ≥10MΩ).
Life cycle test: Continuously drive with rated load (150kg), record motor temperature, battery capacity attenuation and controller failure rate until the first functional failure occurs, and require the mean time between failures (MTBF) ≥ 10,000 hours (FDA medical device standard).
Compliance verification by a third-party certification agency
After passing the internal test, it is necessary to entrust an FDA-approved laboratory (such as UL, TÜV) to conduct full-item testing. For example, the battery system must pass the UN38.3 transportation safety test (mandatory requirement for CE certification), the motor must pass the EN 60034-1 efficiency test (IE3 level or above), and the controller must comply with the EN 61000-6-3 electromagnetic compatibility standard. During the certification process, Suzhou Heins Medical Equipment Co., Ltd.'s products will submit complete technical documents including design drawings, test reports, and material certification to ensure that each link is traceable.
(IV) Quality management system and continuous improvement mechanism
Full process control under ISO standards
Based on the requirements of ISO 13485 (Quality Management System for Medical Devices) and ISO 9001, the production of power drive systems needs to go through:
Design verification (DV): Identify potential risks through FMEA (Failure Mode Analysis) during the R&D stage. For example, when the motor overheating risk level is ≥8, it is necessary to add redundant design of temperature sensors;
Process verification (PV): CPK (Process Capability Index) monitoring of key processes such as welding and assembly is performed, and CPK ≥ 1.33 is required to ensure process stability;
Finished product inspection (FQC): Each power system must pass 100% functional testing (such as no-load current, stall protection response time), and FDA requires a failure rate of ≤ 0.1%.
After-sales data-driven durability optimization
Analyze failure modes through warranty records. For example, if the capacity of a batch of batteries decays too quickly after 1 year of use, it is necessary to trace the electrolyte formula or formation process of the production batch and adjust the parameters in time. A complete customer feedback system has been established to track the entire cycle from parts supply to project implementation. The failure data collected by its after-sales team will be regularly fed back to the R&D department for durability iteration of the next generation of products, ensuring compliance with the FDA's "life cycle management" requirements for medical devices.


 Español
Español Deutsch
Deutsch عربى
عربى